Manisha Dayal and Janet Maldonado
Human
Biology 115A
Winter, 1998
Humans and Viruses
 An Exclusive Interview with
Baruch Blumberg, winner of the 1976 Nobel Prize in
Medicine
An Exclusive Interview with
Baruch Blumberg, winner of the 1976 Nobel Prize in
Medicine
 Introduction to the Family
Introduction to the Family
The name is based on "hepato" (liver) "tropic" (replicates and causes infection in the liver) and DNA virus (its nucleic acid). The Hepadna virus family has the smallest genome of all replication competent animal DNA viruses. The single most important member of the family is Hepatitis B virus (HBV). HBV exhibits an extremely limited host range, infecting only chimps and humans. Our presentation will focus very heavily on Hepatitis B as representative of the Hepadna Family and emphasize human pathogenesis.
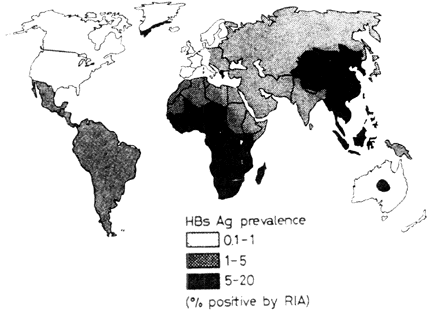
Hepatitis B is extremely prevalent with approximately 300-350 million people worldwide carrying the virus. Chronic infection persists in 25% of children under 5, and 70-90% of infected adults. In Africa and Asia, up to 20% of the population are symptomatic carriers. Many carriers never develop pathogenic symptoms and disease are usually so mild that the actual number of cases could be ten times than that reported. The distribution of Hepatitis B antibody, commonly used as a marker for infection, varies through out the world as does susceptibility to the development of hepatitis disease and liver cancer (Hepatocellular Carcinoma) after exposure to the virus. HCC is the most serious result of infection and makes up 90% of malignant liver tumors. Worldwide HCC is the seventh most common cancer in males and the ninth most common in females. Approximately 500,000 people die a year from the disease. This variation is based on genetic and environmental cofactors. Some individuals carry copies of genes and have lifestyle or natural environmental factors that increases their likelihood of developing hepatitis, HCC or other clinical pathogenesis from infection with Hepatitis B virus. Thus, hepatitis remains a serious health problem in the world.
 Historical notes
Historical notes
Early Mesopotamian civilizations thought that the liver was the basis of life. They were therefore familiar with liver disease and jaundice, the yellow discoloring of the skin and eyes that is a common symptom of hepatitis B infection. By 1885, it was known that hepatitis could be transmitted by syringes and blood transfusions. By 1947, the terms hepatitis A and hepatitis B had been coined by MacCullum to distinguish among a number of outbreaks in the late 1930's. Between the late 1950's and 1970's, Murray had demonstrated that hepatitis disease could be transmitted orally. Research on the family precipitated rapidly in 1963, when Baruch Blumberg, then at the National Institutes of Health and currently at the Fox Chase Cancer Center, was examining thousands of blood samples in search of inherited polymorphisms among different parts of the world. He utilized sera from multiply transfused hemophiliacs, reasoning that such sera would contain genetically polymorphic antibodies. During this investigation, blumberg discovered that a sample from an Australian aborigine contained an antigen, which he later called Australia Antigen and is now called the Hepatitis B surface antigen, which reacted with an antibody in the serum from a hemophiliac subject. By 1968, Prince and Okochi had determined that the Australia antigen was found exclusively in the hepatitis B patients. The characterization of the Hepatitis B surface antigen was a milestone in research because it allowed further study despite inability to isolate the virus. By 1970, Dane et a;. had detected a complete virus particle and in 1981, the first vaccine against hepatitis B called Heptavax was licenced.
 Classification and Taxonomy
Classification and Taxonomy
The hepadna family of viruses consists of five viruses: One human virus, hepatitis B, and four animal viruses: woodchuck hepatitis virus, ground squirrel hepatitis virus, Peking duck hepatitis virus, and other avian hepatitis viruses.
These five viruses are classified together for the following reasons:
1.They are all enveloped
2. They all contain polymerases that can repair the viral DNA genome during replication
3. They produce lipoproteins containing envelope proteins
4. They infect species that are closely related to that of their natural hosts (narrow host range)
5. As the family "hepadna" (for hepatotropic DNA viruses) implies, they can produce chronic infections in liver cells. As stated above, we will focus on the only human virus in the family hepatitis B.
Hepatitis B is characterized by the following properties:
(See genome picture below to facilitate understanding)
1. The hepatitis genome, discovered by William S. Robinson of the Stanford University School of Medicine, is the smallest genome at 3.2 kb long.
2. Genetic information is carried as partially double-stranded DNA.
3. DNA consists of a longer, complete (-) strand with a piece missing at a nicked site, as well as an incomplete (+) strand (about 15-50% of the DNA can be single-stranded)
4. The circular genome is held in a relaxed shape by base pairing of a 240 nucleotide overlap sequence and short direct repeats between the strands at the 5' termini.
5. 5' end of (-) strand has a covalently attached protein whereas 5' end of (+) strand contains an oligoribonucleotide.
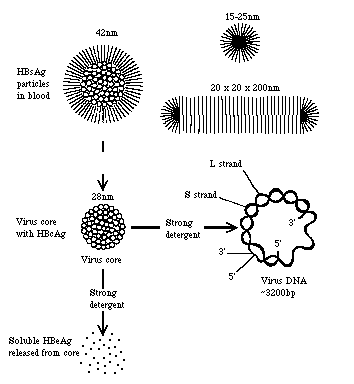
6. Nucleocapsid is icosahedral shaped
7. Nucleocapsid is surrounded by a lipid envelope
8. Nucleocapsid is 27 nm in diameter
9. In the electron micrographic picture, the virion appears spherical and is either 42 nm total in diameter (aka the infectious "Dane particle") or 27 nm. The virus can also appear as a 27 nm rod.
10. Triangulation number=3
11. Replication occurs in the nucleus in the form of DNA-->RNA--> DNA (more on this later)
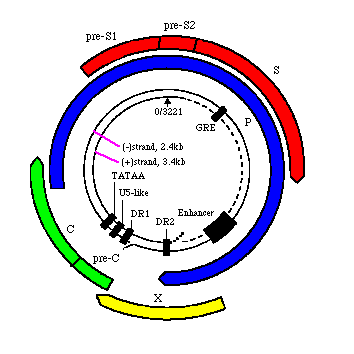
12. Contains four overlapping open reading frames: S, C, P, and X coded on the (-) strand.
13. The S gene encompasses three regions: the pre-S1, pre-S2, and S. Three different proteins are produced by combining these three genes in different combinations: L, the large protein on the envelope is made by encoding the pre-S1, pre-S2, and S genes; middle-sized proteins on the envelope (pre-S2 and S gene); and the major component of the HBsAg, S protein.
The P gene which comprises 80% of the genome encodes a polymerase capable of acting as a DNA polymerase, reverse transcriptase, and RNase H.
The C gene encodes the HBcAg (core antigen).
The X gene encodes a protein that is essential for viral survival and is thought to be associated with transcriptional activation.
14. The synthesis of these proteins is tightly regulated at the transcriptional and translational levels. The two mRNA transcripts are made, one longer than the other. The larger one (3.5 kb), longer than the DNA it is transcribed from due to its 100 bp terminal repeat, encodes both the capsid proteins and the products of gene P. This longer transcript is that which is reverse transcribed to DNA in the Hepatitis replication scheme (see below). The smaller mRNA transcript encodes the middle and major envelope proteins.
The diagram below represents the delineation of the viral particles of the Hepatitis B virus; top: full complete virion; middle: the inner viral core; right: the partially ds genome; bottom: the soluble HBeAg that is released from the core after treatment with SDS.
Hepadnavirus' Unique Replication Scheme
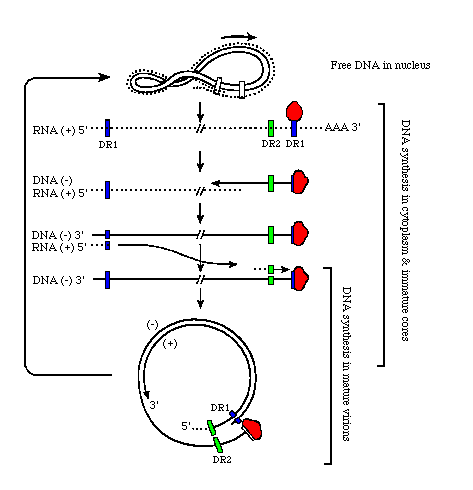
The hepadna virus family has a very unusual mode of replication.
First, the hepatitis B virus enters a liver cell via receptor-mediated endocytosis where it sheds its envelope and the viral DNA is released in the nucleus. (The pre-S1 region facilitates viral entry into hepatocytes.)
Endogenous viral reverse transcriptase then completes the short (+) strand to make the relaxed ds circular configuration. The strands then convalently close on each other by the elimination of three parts (the (+) strand 5' oligoribonucleotide primer, the (-) strand 5' protein, the terminal repeat of the (-) strand) and the subsequent ligation of the ends of the two strands.
The DNA then supercoils to initiate transcription of (+) RNA with the cellular RNA polymerase II.
Three transcripts of mRNA, 2.1, 2.4 and 3.4 kb long, are produced. The l 3.4 kb or "pregenome" (+) RNA is the largest and is longer than the DNA template due to terminal repeat sequences.
In the cytoplasm, the pregenome is translated to yield the core antigens and polymerase and with them it encapsidates itself. (-) DNA is then transcribed within this particle with reverse transcriptase that is produced with the help of the 5' terminal protein.
As transcription occurs, the (+)RNA is chewed away by RNAse H until only a short 5' oligoribonucleotide remains.
This oligo serves as a primer for the complementary (+) DNA. Some of these core virus genomes are sent back to the nucleus to serve as templates for more virus production, however, other are packaged into virions by picking up envelope hepatitis B surface antigen (HBsAg) containing proteins (L, M, and S) in the ER.
Three S antigens are translated from the smaller 2.1 and 2.4 mRNA transcripts, mentioned above. Often times the (+) strand of the DNA is not given enough time to be produced to completion when the virus exits the cell and thus, can vary in length from 1700-2800 base pairs. The virions finally exit the cell by exocytosis.
 genome of
Hepatitis B
genome of
Hepatitis BThe diagram below represents the replication scheme of hepatitis B viral DNA. Key: DR1, 2= 12 bp direct repeat sequences, R=200 bp terminal redundancy in the pregenome mRNA r= short terminal redundancy of (-) DNA strand
Retrovirus Resemblence
There are several feaures of the hepatitis B virus that closely resemble the retrovirus family of viruses, which contains HIV (the virus that causes AIDS):
1) Hepatitis B uses a reverse transcriptase to form DNA from RNA as retroviruses do.
2) The genetic material of both contains a direct repeat.
3) The DNA polymerase and RNAse domains of the reverse trasncriptases are similar.
4)The order and function of the genes in retroviruses gag, pol, env is the same as that in Hepadnavirus genes C, P, and S.
5) Both have oncogenic associations: Hepatitis has been linked to Hepatocellular Carcinoma and HIV to B cell lymphomas.
6) HBV is known to integrate into the host genome in some, but not all, chronically infected patients and HIV always integrates itself.
7) Transmission is similar for both viruses although Hepatitis B is more infectious. (see Transmission section)
 Human Viruses within the Hepadna
family
Human Viruses within the Hepadna
family
There are six members of the Hepadna viral family, no genus sub classification has been established.
Hepatitis B Virus (HBV)
![]() Woodchuck Hepatitis Virus (WHV)
Woodchuck Hepatitis Virus (WHV)
![]() Pekin Duck Hepatitis B Virus (DHBV): isolated by
Baruch
Blumberg
Pekin Duck Hepatitis B Virus (DHBV): isolated by
Baruch
Blumberg
![]() Beechy Ground Squirrel Hepatitis B Virus (GSHV)
Beechy Ground Squirrel Hepatitis B Virus (GSHV)
![]() Heron Hepatitis (HHV)
Heron Hepatitis (HHV)
Hepatitis D
Hepatitis D is the only member of the Deltavirus genus (which is entirely separate from the Hepadna Family) and is a rare virus called a satellite agent. Unlike other satellite viruses, it is replication competent. It does however require Hepatitis B coinfection for transmission because it is found inside Hepatitis B virions. Like Hepatitis B, it is transmitted via blood and fluid. HBV thus acts as a helper for Hepatitis D. Its genome consists of single stranded RNA which is circular and approximately 1678 nucleotides long, the smallest of any known animal virus. It encodes only one or two proteins and there is no sequence homology between its genome and the Hepatitis B genome. The single stranded RNA can basepair on itself once in a circle and fold into a rod like shape, appearing double stranded. The genome codes for its own inner nucleoprotein but the outer coat is made of Hepatitis B surface antigen. Therefore, Hepatitis B provides the necessary outer coat for Hepatitis D. Many disagree about whether Hepatitis D is actually a virus, because like other pathogens called viriods, it has a single strand of covalently closed RNA and self cleaving (with a ribozyme) activity. Coinfection with Hepatitis B and D is more severe than infection with Hepatitis B only, and the incidence of the sudden and highly fatal fulminant Hepatitis is more likely. The likelihood that acute infection will progress to chronic hepatitis or HCC is the same in both cases. Much more severe is superinfection with Hepatitis B and D, where the individual contracts Hepatitis D after Hepatitis B replication has already begun in the liver. By this time, there is plenty of Hepatitis B surface antigen translated that Hepatitis D can use to replicate. Fatality from superinfection can approach 20%. Hepatitis D infection is common in Romania, China, and the western Amazon basin.
 Human Diseases associated with the Hepadna
family
Human Diseases associated with the Hepadna
family
Although Hepatitis B infects hundreds of millions of people, very few develop disease. Variations to host immune response and genetic makeup are key factors. Hepatitis B can cause Hepatitis or acute and latent inflammation of the liver characterized by diffuse and patchy cell necrosis affecting all acini. Primary infection ranges from asymptomatic infection to acute hepatitis. even though infection can be severe, most people clear the infection. Approximately five percent do not and develop persistent infection. Although this is a small number, persistence is important for numerous reasons. First, most of the disease and death from Hepatitis B infection result from persistent infection. Those with persistent infection can die of acute hepatitis within 5 years, or develop HCC in 25 to 30 years.
The disease is often marked by abnormal liver function and jaundice, which is characterized by yellow skin and sclera due to increased levels of the hemoglobin pigment bilirubin released by dying liver cells. Symptoms include flu like syndrome, anorexia, and fever. A number of viruses (and even bacteria) in other taxonomic families cause similar pathology to HBV, but differ in their viral and genomic structure. They are named based on similar clinical presentation. These viruses include Hepatitis A virus (Picorna family), Hepatitis C virus (formerly NonA, NonB nonepidemic hepatitis now in Flavivirus Family), Hepatitis D (currently unclassified in any family, termed Delta agent, acts as an RNA satellite virus to Hepatitis B, Hepatitis E (Calicivirus family), and Hepatitis G (Flavivirus family). It is remarkable that viruses with different nucleic acid, number of nucleic acid strands, and morphology can result in similar diseases.
HBV infection is characterized by the presence of HBsAG (hepatitis B surface antigen also called Australia antigen) , HBcAG (hepatitis B core antigen), and antibodies to these antigens. Antibodies indicate immunity and can be conferred by transfusion, direct Hepatitis B immunoglobulin, pooled gamma globulin, or maternal antibodies or as a result of infection or vaccination. Chronic Hepatitis B carriers can transmit the disease, and can remain both symptomatic and asymptomatic. Why the virus specifically target the liver is not fully understood, although the host's own immune response to the virus has been implicated in the localization of cell death and damage. Ultimately, Hepatitis B infection is highly variable in its clinical manifestation ranging from fulminant hepatitis which is often suddenly fatal to chronic persistent infection which can last for decades. Typically, a person with acute hepatitis will recover completely.
![]() Acute Hepatitis: inflammation of the liver
which occurs rapidly to damage
Acute Hepatitis: inflammation of the liver
which occurs rapidly to damage
![]() Chronic Hepatitis: inflammation of the liver
characterized by persistent infection
Chronic Hepatitis: inflammation of the liver
characterized by persistent infection
![]() Cirrhosis of Liver: degeneration of the liver
with tissue fibrosis and scarring
Cirrhosis of Liver: degeneration of the liver
with tissue fibrosis and scarring
![]() HepatoCellular Carcinoma (HCC): a common
manifestation of chronic HBV carriers. Hepatitis B carriers have a
300 times greater risk of
developing this cancer than uninfected
individuals.
HepatoCellular Carcinoma (HCC): a common
manifestation of chronic HBV carriers. Hepatitis B carriers have a
300 times greater risk of
developing this cancer than uninfected
individuals.
Hepatitis B and Hepatocellular Carcinoma (HCC)
Many believe that hepatitis B is a carcinogen as prevalent and dangerous as tobacco. HCC develops 30 to 50 years after infection with the virus. HCC is a leading cause of death in the world, but especially in Africa, Asia and Southern China where 15% of the population are chronic carriers. 300 million of those infected globally are in Asia. Factors that synergistically act with HBV infection to facilitate progression of HCC are previous liver disease and alcoholism. Hepatitis C has also been strongly associated with HCC, especially primary HCC. The evidence for the connection between Hepatitis B and HCC is epidemiological as the same areas in the world have high percentages of hepatitis B carriers and high rates of HCC. Moreover, integrated viral DNA is often found in cancerous liver cells. The molecular mechanism of Hepatitis B oncogenesis is still unclear. Exposure to large amounts of hepatotoxin aflatoxin B1 (AFB1) appears to be the most important factor. AFB1 increases the probability of a ACG to ACT mutation at the 249th codon of the p53 Tumor Suppressor Gene. This mutation is found in 55% of cases in those areas with high AFB1 exposure, but only 5% of HCC cases in areas with low AFB1 exposure. Currently, there are three models for how Hepatitis B could transform liver cells: it could turn on oncogenes by integrating itself adjacent to those genes, or integrate else where in the genome, or cells regenerating after infection could incorrectly over activate their own growth genes.
 Transmission of Hepatitis B virus
Transmission of Hepatitis B virus
In developing countries with the highest prevalence of HBV, it becomes clear that parenteral transmission is the leading cause of HBV spread. The surface antigen as well as the DNA of the virus are thought to be transmitted through contact of the infected mother's blood with her child during birth (transplacental transmission is more difficult).
Postnatal transmission through breast milk and maternal saliva is also possible. Approximately 50% of the children in countries where HBV is highly prevalent contract the disease from one member of the family that is shedding virus through cuts, sores, or saliva.
In teens and adults, sexual intercourse becomes a significant method of viral transmission. In developing countries, hetero and homosexual transmission plays an even greater role in HBV transmission and perinatal much less.
Contact with contaminated blood is another major way. IV drug abusers, health care workers, renal dialysis patients and to a lesser extent blood transfusion recipients are at risk of contracting the disease. Thus, in developed countries, adults are the target population whereas in developing nations, infants are primarily infected. It is estimated that 1 milliliter of blood from an Hepatitis B positive carrier contains 10 billion particles and that the virus can withstand drying on a surface for over a week.
There have been cases of acute HBV infection that occur sporadically in which the method of acquisition is unknown. Dr. Baruch Blumberg, Nobel Prize winner for the discovery of the first HBV vaccine, researched the possibility of insect transmission by collecting mosquitoes in Uganda and Ethiopia. HBsAg was discovered in both the individual mosquitoes and their eggs. Other studies have shown that the North American bedbug also carries the antigen and that the bedbugs of the beds of carriers of HBsAg actually had a higher infection rate than the bedbugs of non-carriers. This method of transmission has not been further corroborated, however, could help explain the cause of HBV infection in many patients.
 Pathogenesis and Immunity
Pathogenesis and Immunity
The Hepadnaviruses are specific for liver cells, that is, they exhibit hepatotropism. This cell preference may be caused by a regulatory gene in HBV, liver cell differentiation state, or a viral gene enhancer induced by hormones. However, HBV has also been detected in bile duct epithelium, pancreatic acinar cells, B lymphocytes, monocytes, and circulates free in the plasma.
The Hepatitis core antigen has been detected by immunofluorescence in both the cytoplasm and nucleus in the cells of both acute and chronic hepatitis patients. The hepatitis B surface antigen has been seen only in the cytoplasm and blood along with whole virions, viral DNA, and viral DNA polymerase. In fact, in chronically infected individuals the surface antigen is found in very high concentrations (1013 particles/ml) in the serum for years to life; whole infectious virions are at a much lower level. During acute infection or the "high" replicative phase, the DNA made from replication cycles back into the nucleus. However, integration of HBV DNA has also been observed in chronic carriers of the virus during the "low" replication phase. The only transcript produced by the integrated DNA is subgenomic mRNA or the hepatitis B surface antigen and it can not replicate since it does not have the recognition site for its reverse transcriptase. Thus, this noninfectious protein is the main antigen that is present in chronic carriers of the virus.
The immune response to viral infection is principally cell-mediated by T8 cytotoxic cells that recognize viral peptide/MHCI presented on the surface of infected liver cells. Although liver cells do not usually have a high number of MHC class I molecules, the interferon produced by viral infection upregulates the expression and activates more Tc and nonspecific Natural Killer cells. To fight off primary infection, T8 cells recognize the peptides of the core and serotype e antigens of hepatitis. The surface antigen and pre-S regions provide the immunogenicity during reinfection when neutralizing antibodies attack and CD4 T cells help. These antibodies are also cross reacting and thus, are capable of attacking other serotypes. Interestingly, production of the noninfectious smaller 28 nm proteins (middle and major) is extremely unregulated (105 particles are detected in serum) during infection compared to the large proteins present only on the surface of full infectious virions. This is thought to occur as a decoy; the smaller particles soak up most of the neutralizing antibodies produced in order to promote the survival of the "real" infectious virions. As stated before, gene S is one of the most immunogenic proteins of the virus and is thought to be expressed at very high levels on liver cells. It was recently discovered by Christel Pourcel at the Pasteur Institute at the University of Paris, that expression of gene S is unregulated in the presence of steroids. This finding has helped to understand why males (who have a higher level of steroids) carry an increased risk of developing chronic HBV and hepatocellular carcinoma.
Acute infection is usually successfully defeated by the immune response of the host, however, in 5-10% of the total population and >90% of infants afflicted with Hepatitis, the virions remain in the body as a persistent infection (these are known as the chronic carriers of HBV). Carriers produce both infectious and noninfectious particles (many more noninfectious ones are made as explained above), which cruise around in the plasma, and to a lesser degree in the semen and saliva. Because so much of these smaller proteins are made, chronic carriers are frequently afflicted with "immune-complex" diseases in kidneys and arterioles. The virus is thought to remain persistent for three reasons: 1) T8 cells recognize only HBcAg and HBeAg and neither of these are present in the subgenomic mRNA produced by transcription of the integrated DNA during chronic infection. 2) the liver cells that contain low replicating DNA of the virus do not express much class I MHC to present peptides to T8 cells. 3) It is believed that some carriers (especially those who do not respond to post HBV vaccination) have built a tolerance to HBV by the action of suppressor T cells.
 Management and therapy of infection
Management and therapy of infection
After infection with hepatitis B, patients have various options for treatment, the most effective of which being post-prophylaxis immunization. Those exposed receive Hepatitis B immune globulin isolated from the blood of individuals who have anti-Hepatitis B antibodies. This method is even more effective in preventing the negative ramifications associated with infection when both antibodies and the active vaccination are administered to the individual.
Post-transfusion HBV infection has been markedly reduced by routine screening of donors by immunoassay or radio immunoassay. Not much can be done to treat acute viral hepatitis and restrictions on diet and activity are without scientific basis.
Chemotherapy is the main source of treatment for chronic carriers of the virus that develop chronic (aggressive) hepatitis. The principle benefit of these drugs is to ameliorate and manage the complications associated with the disease, not to cure it. The reverse transcriptase and the DNA polymerase inhibitors, AZT and acyclovir respectively, have not been shown to have much promise in the inhibition of HBV replication. Ganciclovir in combination with foscarnet has been shown to be the most effective treatment for those afflicted with chronic hepatitis, since studies show that a significant amount of viral replication is halted. Nucleoside analogs such as penciclovir, that exploit the nonspecific polymerase of the virus have been shown to inhibit HBV replication in the lab. Nalidixic acid is being studied as a DNA gyrase inhibitor that could interefere with the initiation of viral transcription by changing the DNA structure. Interferon is also being used as treatment but has many negative aspects; i.e. it must be administered subcutaneously (5-10 million units injected three times a week), is expensive, and produces flu-like side effects. However, it has been proven to reduce inflammation and aminotransferase levels after a 4-6 month treatment in a very select group of chronic hepatitis patients (those in which the virus is in the low to high replicative transition phase). This improvement have only been seen in 50% of the patients and the benefits disappear right after treatment is curtailed.
 Prevention and Eradication of Hepatitis B
Prevention and Eradication of Hepatitis B
Prophylaxis or protection from the virus causing infection induces both passive and active immunity. For passive immunity, Hepatitis B Immunoglobulin is given. This allows the individual to neutralize as much virus in the serum as possible. If the virus is reduced to significantly low levels, active immune responses including T cell responses could control or prevent latent and persistent infection. Vaccination with either purified or recombinant antigen induces the individuals own antibody and T cell response. However, 3-4% of vaccinees do not mount a successful immune response to the vaccine and are not protected from infection. The failure of the vaccine may be due to adjuvants, the chemicals given with the vaccine, and genetic polymorphisms. Just as some individuals are more likely to develop acute hepatitis rather than asymptomatic infection or more likely to have infection progress to Hepatocellular carcinoma than remain persistent, some of those vaccinated are protected and others are not. Other factors that influence the immune response to the vaccine are age and weight of the host, smoking, and strength of immune system.
Other preventative measures include screening prenatal infants and mothers and postnatal infants with enzyme immunoassay or radioassay and providing passive prophylaxis with HBIG or post exposure vaccination. Prenatal screening as a tremendous benefit in the long run. If more cases of chronic carriers (including those chronic carriers who are asymptomatic but continue to spread the virus to others) can be avoided by screening and vaccinating very early in life., the rates of hepatitis, cirrhosis and hepatocellular carcinoma can be greatly reduced.
Another crucial element in preventing the spread of Hepatitis B infection is testing the blood supply to avoid giving virus contaminated blood to those who are seronegative. In the US, Hepatitis B is most commonly transmitted through blood transfusions and IV drug users. IV drug users are the largest group of carriers in the US. Currently, every blood bank in the United States screens its blood for the presence of the Hepatitis B antibody. It is presumed that if the antibody is found, the blood donor has the virus. Health care workers who come in contact with blood frequently are also at increased risk for contracting the virus. These individuals especially should take special precautions when handling blood and be vaccinated. Secondly, attempts at preventing the reuse and sharing of syringes by IV drug users can reduce the spread of Hepatitis B as well as HIV. Unfortunately high cost and logistics for screening prenatally and providing prophylaxis are impediments to infection prevention in the developing world, where hepatitis infections and deaths are the highest.
The screening for Hb antigen in blood banks has greatly reduced transfusion hepatitis. This should continue. IV drug users should also be educated about the risk of transmission through needle sharing. Needle exchange programs have been successful. Transmission via sexual contact is more difficult to address but can be decreased by monogamy, screening of positive sexual partners, vaccination of partners of known carriers, avoiding use of carrier's fomites (toothbrush, razor, eating utensils). Perinatal transmission can be curtailed by postnatal vaccination and administration of HBVIG. Those high risk individuals in constant contact with blood or serum of carriers should wear gloves and take special precautions when using needles.
Worldwide eradication will be exceedingly difficult, despite very effective vaccines. First, many in the developing and developed world do not perceive Hepatitis B as a serious disease. They should be educated about the connection between Hepatitis B and liver cancer as well as the widespread prevalence of the virus in asymptomatic carriers. Also, Hepatitis B does not always cause clinically apparent disease, and much of the perinatal and sexual transmission is accounted for by asymptomatic carriers. This makes identifying those who should be vaccinated or treated with antivirals before they transmit to many contacts very difficult. The technology to do this does not exist in the developing world, where Hep B carriage is the most common. The only carriers of the virus are humans and chimps, meaning that it is not necessary to isolate and eliminate the virus in other natural reservoirs. This facilitates eradication of the virus. Although vaccination is effective, it is expensive, making it more difficult to administer on a global scale and in a universal fashion. Nonetheless, the vaccine has few side effects, has proven very effective. Full efficacy requires subsequent booster shots. Despite cost, delivery will be difficult because it requires cold storage and syringes. This is difficult in tropical climates of Africa and Asia and stands in sharp contrast to small pox eradication where a freeze dried vaccine was administered using a bifurcated needle. In general, one hindrance to eradication is that Hepatitis carriers are much more common in Asia and Africa than in the rest of the world. A lack of infrastructure, accurate and recent records at blood banks, surveillance and containment of carriers capability, issues of nonexistent transportation and communication all make universal vaccination of neonates and other at risk groups exceedingly difficult. Furthermore, developed countries may not see any incentive to fund vaccination and build infracture to facilitate eradication in these countries. At the same time, if the Hepatitis B vaccine is perceived as an anti-cancer vaccine that can prevent Hepatocellular Carcinoma, a common global cancer, and save tremendous financial and human loss in the future these countries may be more willing to organize and carry out global vaccination campaigns.
 Vaccination
Vaccination
The first Hepatitis B vaccine was marketed by Merck Sharp and Dohme as Heptavax-B and licensed in 1981. It is based on extraction and purification of the HBsAG antigen from the serum of chronic carriers and thus also called a plasma derived vaccine. Notably, it was the first subunit vaccine approved by the FDA. A subunit vaccine is one that contains a small portion of the virus rather than attenuated virus. With a subunit vaccine, there is less possibility of viral infection from the virus because the complete replication competent virus is not injected.
The most recently FDA approved vaccine is based on cloned copies of HBsAG gene in yeast and manufactured as Recombivax HB by Chiron corp. and Merck and Engerix-B by SmithKline Biologicals. This is the world's first ever recombinant vaccine. These are also subunit vaccines. Both vaccines have proved highly efficacious and safe. Protective levels of antibody are elicited in over 90%of recipients. Both have rare serious side effects .
Vaccination strategies vary widely from country to country. In the developing world, the first priority must be to stop perinatal transmission by vaccinating all infants at birth. The vaccine could be combined with other ones to ease delivery. Developed nations on the other hand vaccinate at risk individuals (newborns from Ab positive mothers, IV drug users, travelers to endemic regions, and hemophiliacs), but these groups are often hard to find and vaccinate. This is why carrier rates are increasing in these countries. Universal vaccination of all infants would eliminate this difficult tracking down process.
 Great Links to Hepatitis B
pages
Great Links to Hepatitis B
pages
 Bibliography and References
Bibliography and References
![]() Textbooks:
Textbooks:
David White and Frank Fenner.
Medical
Virology, fourth edition,
Academic Press, San Diego: 1994. pages 358-379
Bernard Fields and D.M. Knipe and P.M. Howley et. al. Virology, third edition, Lippincott-Raven, Philadelphia: 1996, pages 2739-2790.
![]() Primary
References:
Primary
References:
Blumberg, Baruch. "Australia Antigen and the Biology of Hepatitis B," The Nobel Foundation, 1997.
Blumberg, Baruch. "Hepatitis B virus, the vaccine, and the control of primary cancer of the liver," Proceedings of the National Academy of Sciences USA, vol 94, pages 7121-25, July 1997.
Seeger, C., Ganem, D., Varmus, H. "Biochemical and genetic evidence for the Hepatitis B replication strategy," Science 232: 447-84, 1986.
Tiollais, Pierre and Buendia, Marie-Annick. "Hepatitis B Virus," Scientific American, vol 264, no. 4, April, 116-23, 1991.
 Brought to you
by Manisha Dayal, Junior, double major in Human Biology
(Concentration in Molecular Neuroscience) and English and Janet
Maldonado, senior, major in Human Biology at
Stanford University.
Brought to you
by Manisha Dayal, Junior, double major in Human Biology
(Concentration in Molecular Neuroscience) and English and Janet
Maldonado, senior, major in Human Biology at
Stanford University.
![]() Created: February
1, 1998
Created: February
1, 1998
Last modified: March 8, 1998
Acknowledgements: We would like to thank our friends Greg Schwartz and Andrew Carhart for their assistance